Paper accepted in RSC Advances!
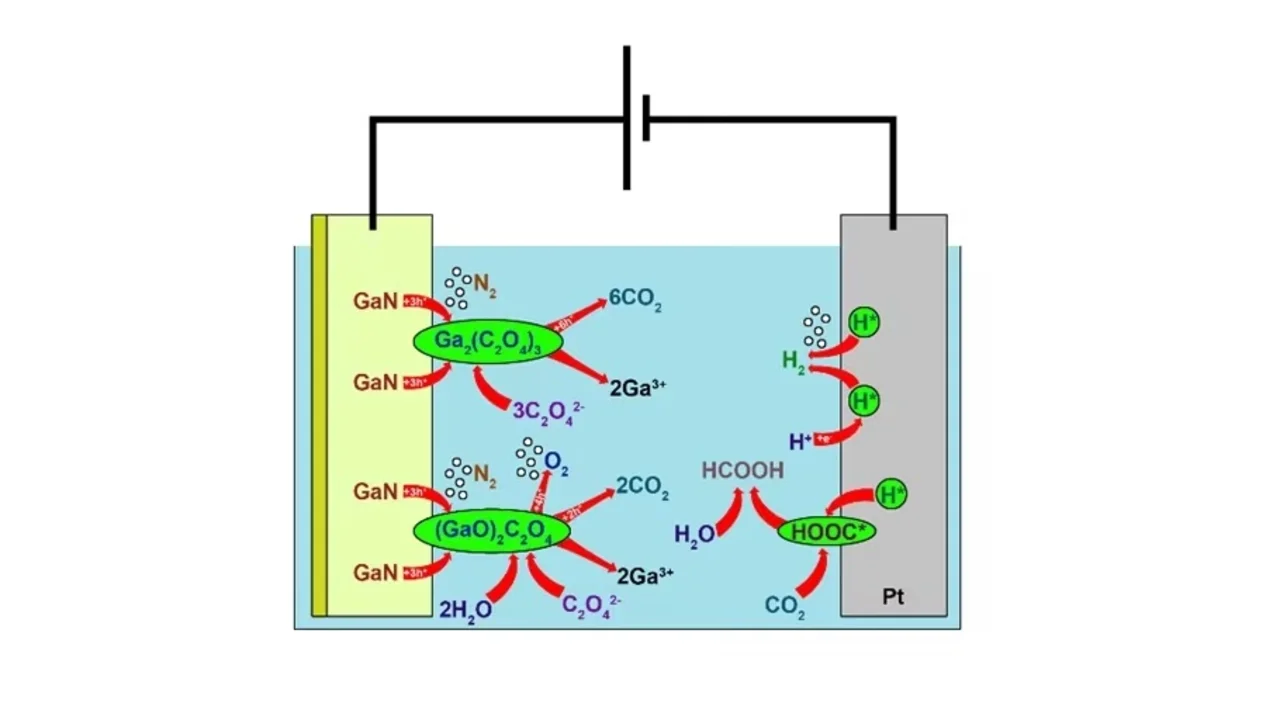
The paper reports the investigation of the wet electrochemical etching of n-GaN films in oxalic acid. The results of a number of quantitative physicochemical analysis methods of the products indicated a 6-electron nature of the etching reaction and fully revealed the mechanism of EC oxidation of n-GaN, which includes the formation of intermediates formed by adsorption of the solvent on GaN surface. Clear understanding of this reaction creates a solid foundation for the investigation of transformations of more complicated III-nitride systems for low-damage and high-precision processing of nitride-based devices.
About
[Abstract] We studied the wet electrochemical etching of n-GaN films in oxalic acid. The electrooxidation process occurs in potentiostatic mode in the voltage range of 5 to 20 V. We described the formation of porous n-GaN layer structures in several ways. Firstly, we observed the microphotographs of the cross section to characterize the nanostructure. Secondly, we examined the reaction products in liquid phase by ICP-OES and TOC-TN methods, while vapor phase products were registered by a gas chromatography. Finally, according to the product data analysis, we demonstrate a mechanism of the electrochemical oxidation of n-GaN in oxalic acid, which involves 6 electrons.



